
Let’s see some interconversion from 1 atm. Note: We know the interconversion between the units to do various calculation problems. So we can expresses the equation of pressure as, We know that pressure is the force exerted in a specific area and it is calculated by dividing the force applied by the area on which the force was applied. So before going into the solution part of the question, let’s discuss some concepts about pressure and its unit. In the question, it is asked that we have to convert the given value of unit in mm Hg to atmospheres of pressure.The question is simply about te unit conversion of values between the units of pressure, We should be familiar with 1 atm value equal to how much value of mmHg. It's used in British and American society and that's about it.Hint: The approach for the question should be done by focusing on the unit conversion of the value from mmHg to atmospheres of pressure in atm. (720.0 mmHg) (101,325 Pa / 760 mmHg) = 9.599 x 10 4 PaĬomment: Pounds per square inch is not used in scientific circles. Express this pressure in atmospheres, kilopascals, torrs, pounds per square inch, and pascals. 101.325 kPa = 101,325 Pa.Įxample #12: A student reads a barometer in the laboratory and finds the prevailing atmospheric pressure to be 720.0 mmHg. KPa (kilopascals) is the more commonly used unit, but Pa is used from time to time. Don't tell 'em some guy on the Internet (me!) said they were wrong. If your instructor insists in using Torr, just follow his/her practice. By the way, it is a lowercase t, not a capital, as in Torr. It was intended as a replacement for mmHg, however, mmHg did not fade away. Notice that the kPa will cancel, since one is in the numerator and one is in the denominator, leaving mmHg as the unit on the answer. Notice that the mmHg will cancel, since one is in the numerator and one is in the denominator, leaving kPa as the unit on the answer. In this conversion, both 760.0 and 101.325 will be involved and the location of each (numerator or denominator) will depend on the conversion. The 1 was assumed to be present.īy the way, the 1 (as in 1 atm) has no influence on significant figures. For example, 760.0 mmHg / 1 atm in examples 1 and 2. The conversion examples above are examples of a one being involved. This situation is slighly unusual because most conversions involve a one, usually in the denominator. Converting between millimeters of mercury and kilopascals.ħ60.0 mmHg equals 101.325 kPa, so both values will be involved. The answer is rounded to four significant figures. Notice that the kPa values cancel and the atm, in the denominator of the denominator, moves to the numerator. Solution: divide the kPa value by 101.325 kPa / atm. In that case, think of the 1 as being understood to be there. Sometimes the 1 in front of atm is eliminated.
Notice that the atm values( one in the numerator and one in the denominator) cancel, leaving kPa. Solution: multiply the atm value by 101.325 kPa / atm. One atm equals 101.325 kPa, so there will be a multiplication or division based on the direction of the change. Converting between atmospheres and kilopascals. Note also that the answer of 0.980 atm has been rounded off to three significant figures. Notice that the mmHg values cancel and the atm, in the denominator of the denominator, moves to the numerator. Solution: divide the mmHg value by 760.0 mmHg / atm Notice that the atm values (one in the numerator and one in the denominator) cancel, leaving mmHg. Solution: multiply the atm value by 760.0 mmHg / atm. equals 760.0 mm Hg, so there will be a multiplication or division based on the direction of the change. Converting between atmospheres and millimeters of mercury. This is an unfortunate situation, but we cannot change it.
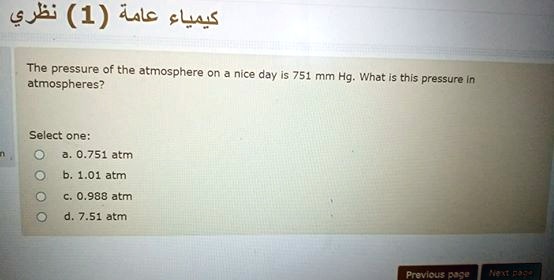
There are three different units of pressure used in chemistry. Here is a repeat from the "Four Variables" file: Our conversions provide a quick and easy way to convert between Pressure units. Converting between Units of Pressure: atm, mmHg and kPa Converting between Units of Pressure: atm., mmHg and kPa Online calculator to convert millimeters of mercury to atmospheres (mmHg to atm) with formulas, examples, and tables.


 0 kommentar(er)
0 kommentar(er)
